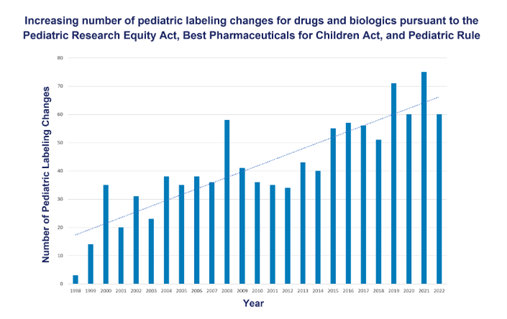
“A pediatric labeling change refers to any update to a product’s labeling to add information about safety, effectiveness, or dosing for children.”
As you can imagine, the number of labeling changes to pediatric medications has increased over the years due to more research, new findings, and better information. There are a number of legislative initiatives, including the Pediatric Research Equity Act (PREA) of 2003, the Best Pharmaceuticals for Children Act (BPCA) of 2002, and the Pediatric Rule of 1998, that have helped increase the submission of pediatric studies to the FDA and have resulted in new treatment options for children.
You can view all changes to pediatric labels through the legislative initiatives noted above here.
Pragmatyxs works closely with our pharmaceutical partners to ensure the correct labels, with the accurate and most up-to-date information are on their products. Through automated labeling solutions, changes to labels are as easy as the click of a button, and we partner with a number of labeling solution providers to ensure the right option is available.