 We have heard a lot through the news lately of drug shortages, mostly over-the-counter cold and flu medications, but what is the process when prescription drugs are in short supply? The FDA works closely with the pharmaceutical industry and stakeholders to “prevent drug shortages and lessen the impact on patients anytime there is a delay in the availability of the medicines you need.” There are some situations that are out of the control of the FDA, no matter how in step they are with drug companies. Some of the factors that can contribute to shortages are:
We have heard a lot through the news lately of drug shortages, mostly over-the-counter cold and flu medications, but what is the process when prescription drugs are in short supply? The FDA works closely with the pharmaceutical industry and stakeholders to “prevent drug shortages and lessen the impact on patients anytime there is a delay in the availability of the medicines you need.” There are some situations that are out of the control of the FDA, no matter how in step they are with drug companies. Some of the factors that can contribute to shortages are:
- Breakdowns in the manufacturing line
- Product quality problems including recalls and discontinued product
- An unexpected surge in demand
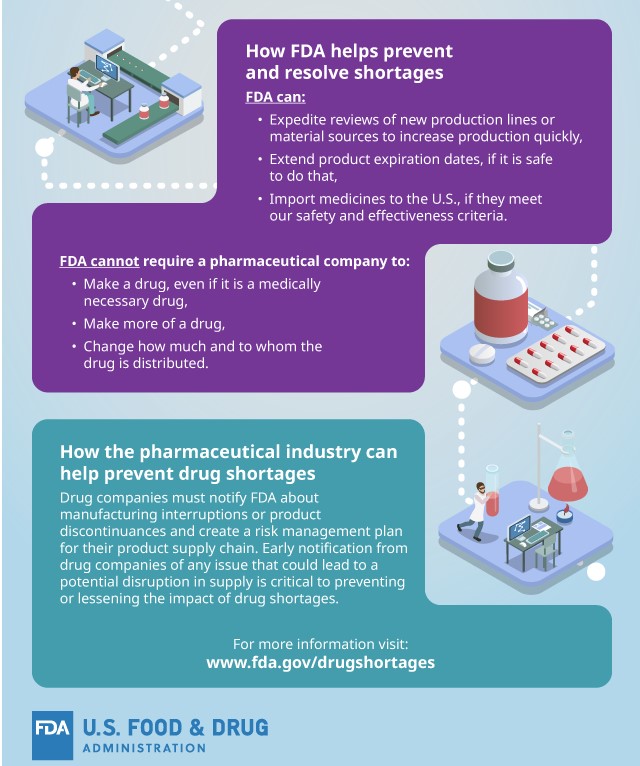
Beyond the activities outside the control of the FDA and sometimes the pharmaceutical companies themselves, there are a few steps that the FDA takes to help prevent and resolve shortages. Some of those steps are:
- Expedite reviews of new production lines or material sources to increase production quickly.
- Extend product expiration dates, if safe to do so.
- Import medicines to the U.S. if they meet our safety and effectiveness criteria.
Even as a governmental agency, there are a few things that the FDA cannot require a pharmaceutical company to do, which are: make a drug, even if it is a medically necessary drug, make more of a drug or change how and to whom the drug is distributed.
Pragmatyxs works closely with our pharmaceutical partners to ensure their labeling systems are ready to accommodate changing needs like an expiration date change or product requiring information submission in a computer-readable structured labeling format to the Global Unique Device Identification Database (GUDID). Pragmatyxs can assist your organization in delivering validated, compliant solutions for barcode printing, validation, measurement, and audit of all label functions in your supply chain.